Have you taken a look at your sycamore lately? Seeing any leaves this spring? Looks like a little problem!

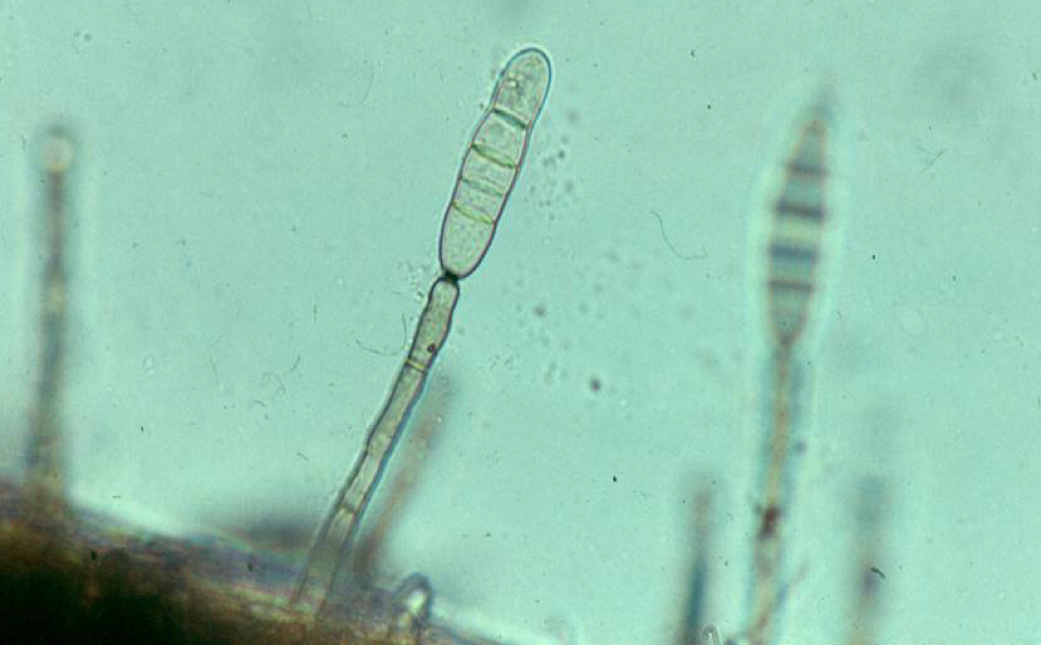
Classic angular leaf spot and twig blight of sycamore anthracnose. Photo: Richard Buckley, Rutgers PDL
Samples of deciduous shade trees diagnosed with anthracnose have steadily found their way into Rutgers Plant Diagnostic Laboratory this spring. Anthracnose is a common fungal disease of shade trees that results in angular leaf spots, cupping or curling of leaves, and premature defoliation. Green stems and twigs can become infected, causing twigs to dieback and overwintering cankers to form. We’ve had samples of sycamore and London plane tree, but have also had ash, beech, maple and a bunch of oaks.
The fungi responsible for anthracnose in shade trees are all unique. The fungus that attacks sycamore is not the same as the fungi that cause ash, beech, dogwood, maple, or oak anthracnose. Each tree species has a specific fungus causing its own anthracnose disease, so for example, the beech anthracnose fungus does not infect oaks and the oak anthracnose fungus does not infect maples and so on.

Marginal necrosis and irregularly-shaped, angular leaf spots on silver maple (Acer saccharinum). Photo: Sabrina Tirpak, Rutgers PDL
Infections by anthracnose causing fungi are favored by cool, wet conditions during the budbreak period in the spring. When the weather favors one of these fungi, it generally favors all of them, so we see the disease to some degree on many different hosts. Anthracnose fungi survive winter in buds, small twig cankers, or fallen leaves depending on which types of trees and fungi are involved. In the spring, the spores are moved by wind and water to newly forming leaves. The longer the weather conditions remain cool and wet, the more damage one can expect. Once the weather becomes dry and the leaves mature, the disease cycle ends and the tree will replace lost leaves with new ones.
Anthracnose can cause a very visible leaf lesion, and depending on the fungus/tree species dynamic, may defoliate the tree. Sycamore anthracnose causes a very significant defoliation, while beech anthracnose rarely does. Furthermore, it is not uncommon for the causal fungus to kill the buds before they open in the spring. It’s also not uncommon for the fungus to kill new green stems and twigs. Although these diseases often seem severe, they have little long-term impact on overall tree health. Leaves and buds damaged early in the season are often replaced by mid-summer.

Small spots with yellow borders and distorted leaves, caused by oak anthracnose. Photo: Sabrina Tirpak, Rutgers PDL
So, what do we do about anthracnose? Not much, really! Rake and remove fallen leaves. Improve plant vigor with fertilization and irrigation in times of drought, and prune all of the dead and dying limbs. Fungicides can be used to prevent the problem in high-value trees. Begin treatments at budbreak to protect the new growth and repeat the treatments 2-3 times at the label specified intervals. Proper timing and good coverage are essential, which will necessitate a licensed professional applicator to make the applications.
By the way, it is too late for fungicide protection this season!
Fungal diseases in most ornamental plants can be prevented with applications of one or a combination of the following active ingredients: FRAC M3 mancozeb; FRAC M5 chlorothalonil; FRAC 1 thiophanate-methyl; FRAC 3 metconazole, myclobutanil, propiconazole, tebuconazole, triademefon, triflumizole; FRAC 7 boscalid, flutolanil, oxycarboxin; FRAC 11 azoxystrobin, fluoxastrobin, kresoxim-methyl, pyraclostrobin, trifloxystrobin; FRAC 12 fludioxonil; and/or FRAC 19 polyoxin-D. Be sure to follow all label specifications for the host plant, the specific diseases controlled, as well as rates, dilution, and timing.